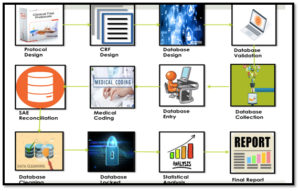
Documentation plays a vital role in clinical research. It validates how authentic the research data was collected and verify the result of data.
There are many stakeholders in clinical research-participants, sponsor, regulatory authority, ethics committee each playing an integral role for thru drug to come in the market for sale.
In clinical research “if is not documented, it is not there”!
Documentation of the activities in clinical research to ensure the quality. In good clinical practice, essential documents individually and collectively permit evaluation of the conduct of a study and the quality of the data produced.
Good documentation practice describes procedure for correct documentation.

- All documentation entries shall be done with black ink (ball point) and legible handwriting.
- Do not leave any document incomplete. If not applicable write not applicable (NA).
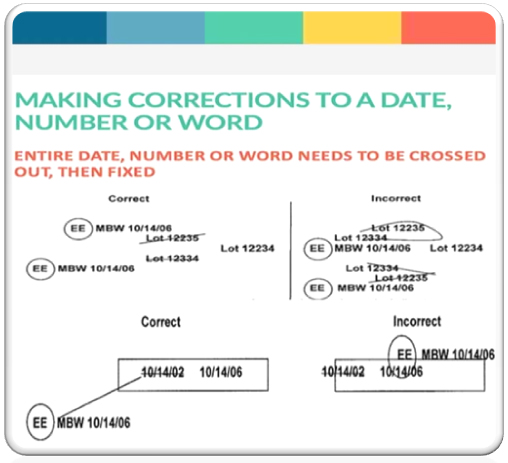
- Do not use correction fluid in any documents.
- All personnel should ensure to avoid error during data entry.
- In case error has occurred. do not overwrites wrong entries, cross with a line permit the reading of original entry. Clearly write the correct entry near the cross out. initial/sign and put date on which correction was made.
Clinical research documentation involves a variety of documents from various sources is often completed by several people.
Inadequacies in documentation could be a result of lack of training and experience in understanding of clinical and document requirements.
Unmonitored medical records are discovered at the time of audit/inspection, such as diaries of coordinator, in patient records of hospital for simple reason as negligence

How can documentation be improved?
The principal investigator should delegate responsibility to staff adequately trained in protocol and gcp.
Particular training should be provided on alocoa+ and other good documentation practice
Alcoa+
- Attributable means information is captured in the record so that it is uniquely identified as executed by the originator of the data (e.g. A person or a computer system).
- Legible, traceable and permanent. The terms legible and traceable and permanent refer to the requirements that data are readable, understandable, and allow a clear picture of the sequencing of steps or events in the record so that all gxp activities conducted can be fully reconstructed by the people reviewing these records at any point during the records retention period set by the applicable gxp.
- Contemporaneous data are data recorded at the time they are generated or observed.

- Original data include the first or source capture of data or information and all subsequent data required to fully reconstruct the conduct of the gxp activity.
The gxp requirements for original data include the following:
- Original data should be reviewed;
- Original data and/or true and verified copies that preserve the content and meaning of the original data should be retained;
- As such, original records should be complete, enduring and readily retrievable and readable throughout the records retention period.
- The term “accurate” means data are correct, truthful, complete, valid and reliable.
Implicit in the above-listed requirements for alcoa are that the records should be complete, consistent, enduring and available (to emphasize these requirements, this is sometimes referred to as alcoa-plus).
Site should develop sop for good documentation,the sop should be shared with the sponsor/cro and agreed upon before start of trial.
- Original data include the first or source capture of data or information and all subsequent data required to fully reconstruct the conduct of the gxp activity.
The gxp requirements for original data include the following:
- Original data should be reviewed;
- Original data and/or true and verified copies that preserve the content and meaning of the original data should be retained;
- As such, original records should be complete, enduring and readily retrievable and readable throughout the records retention period.
- The term “accurate” means data are correct, truthful, complete, valid and reliable.
Implicit in the above-listed requirements for alcoa are that the records should be complete, consistent, enduring and available (to emphasize these requirements, this is sometimes referred to as alcoa-plus).
Site should develop sop for good documentation,the sop should be shared with the sponsor/cro and agreed upon before start of trial.

Before start of trial all technical aspect for ecrf, fax, printer should be clarified and issues resolved.
Sponsor /cro also play an important role in ensuring quality of source documentation.
Conclusion
The documentation should tell the entire story and speak for itself for the study participants journey as it happened to auditor/inspector thus making a complete picture and forming a strong foundation for clinical research.