Skip to content
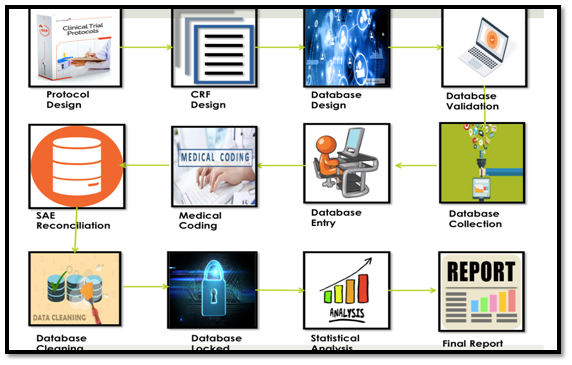 Steps in CDM Self-evident corrections are changes to data or query resolution, that can be easily corrected on the basis of information entered on the Case Record Form (CRF), without sending a query to the site. Some of the most common self-evident corrections are spelling errors. A section should specify the criteria for self-evident corrections, [...]
Steps in CDM Self-evident corrections are changes to data or query resolution, that can be easily corrected on the basis of information entered on the Case Record Form (CRF), without sending a query to the site. Some of the most common self-evident corrections are spelling errors. A section should specify the criteria for self-evident corrections, [...]
 Softwares like Argus, ArisGlobal, and PvNET are used in Pharmacovigilance. VigiFlow, VigiBase used in post marketing surveillance VigiBase is a WHO's global Individual Case Safety Report (ICSR) database that contains ICSRs submitted by the participating member states enrolled under WHO’s international drug monitoring programme. Uppsala Monitoring Centre (UMC) in collaboration with ‘’Swissmedic’’ has developed ‘’VigiFlow’’, a web-based ICSR [...]
Softwares like Argus, ArisGlobal, and PvNET are used in Pharmacovigilance. VigiFlow, VigiBase used in post marketing surveillance VigiBase is a WHO's global Individual Case Safety Report (ICSR) database that contains ICSRs submitted by the participating member states enrolled under WHO’s international drug monitoring programme. Uppsala Monitoring Centre (UMC) in collaboration with ‘’Swissmedic’’ has developed ‘’VigiFlow’’, a web-based ICSR [...]
 Good pharmacovigilance practices (GVP) are the minimum standard requisite to monitor safety of medicines that is exposed to the public. These are set of procedures that expedite the practice of pharmacovigilance. These practices are used in relation to medicines that are legalized at the national level. These standard procedures ensure authorization holders for an adequate [...]
Good pharmacovigilance practices (GVP) are the minimum standard requisite to monitor safety of medicines that is exposed to the public. These are set of procedures that expedite the practice of pharmacovigilance. These practices are used in relation to medicines that are legalized at the national level. These standard procedures ensure authorization holders for an adequate [...]
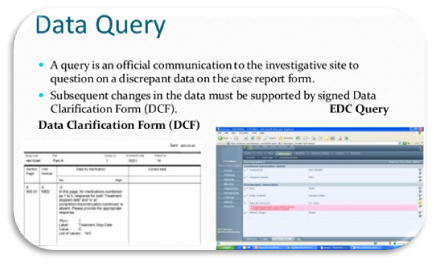 A Data Clarification Form (DCF) or Data Query Form (DQF) is a questionnaire specifically used in clinical research. The DCF is the primary data clarification tool from the trial sponsor or Contract Research Organization (CRO) towards the investigator to clarify discrepancies and ask the investigator for clarification. The DCF is part of the data validation process in [...]
A Data Clarification Form (DCF) or Data Query Form (DQF) is a questionnaire specifically used in clinical research. The DCF is the primary data clarification tool from the trial sponsor or Contract Research Organization (CRO) towards the investigator to clarify discrepancies and ask the investigator for clarification. The DCF is part of the data validation process in [...]
 Documentation plays a vital role in clinical research. It validates how authentic the research data was collected and verify the result of data. There are many stakeholders in clinical research-participants, sponsor, regulatory authority, ethics committee each playing an integral role for thru drug to come in the market for sale. In clinical research “if is [...]
Documentation plays a vital role in clinical research. It validates how authentic the research data was collected and verify the result of data. There are many stakeholders in clinical research-participants, sponsor, regulatory authority, ethics committee each playing an integral role for thru drug to come in the market for sale. In clinical research “if is [...]
 Firstly, we must grasp that the phrase used for clinical trial patient is preferred as participant, they are voluntarily participating in the study and may be healthy or diseased volunteers. By voluntariness it is meant they can withdraw from the study at any given point of time. In clinical trial, the safety of the participant [...]
Firstly, we must grasp that the phrase used for clinical trial patient is preferred as participant, they are voluntarily participating in the study and may be healthy or diseased volunteers. By voluntariness it is meant they can withdraw from the study at any given point of time. In clinical trial, the safety of the participant [...]
 Firstly let us understand what is clinical trial Clinical trial in simple terms validates the safety and efficacy of the drug to enter the market for sale.Clinical trial undergoes various phases increasing the number of participant to the next step. Epidemic-The occurrence of more cases of a disease that would be expected in a communt [...]
Firstly let us understand what is clinical trial Clinical trial in simple terms validates the safety and efficacy of the drug to enter the market for sale.Clinical trial undergoes various phases increasing the number of participant to the next step. Epidemic-The occurrence of more cases of a disease that would be expected in a communt [...]
 Steps in CDM Self-evident corrections are changes to data or query resolution, that can be easily corrected on the basis of information entered on the Case Record Form (CRF), without sending a query to the site. Some of the most common self-evident corrections are spelling errors. A section should specify the criteria for self-evident corrections, [...]
Steps in CDM Self-evident corrections are changes to data or query resolution, that can be easily corrected on the basis of information entered on the Case Record Form (CRF), without sending a query to the site. Some of the most common self-evident corrections are spelling errors. A section should specify the criteria for self-evident corrections, [...]  Softwares like Argus, ArisGlobal, and PvNET are used in Pharmacovigilance. VigiFlow, VigiBase used in post marketing surveillance VigiBase is a WHO's global Individual Case Safety Report (ICSR) database that contains ICSRs submitted by the participating member states enrolled under WHO’s international drug monitoring programme. Uppsala Monitoring Centre (UMC) in collaboration with ‘’Swissmedic’’ has developed ‘’VigiFlow’’, a web-based ICSR [...]
Softwares like Argus, ArisGlobal, and PvNET are used in Pharmacovigilance. VigiFlow, VigiBase used in post marketing surveillance VigiBase is a WHO's global Individual Case Safety Report (ICSR) database that contains ICSRs submitted by the participating member states enrolled under WHO’s international drug monitoring programme. Uppsala Monitoring Centre (UMC) in collaboration with ‘’Swissmedic’’ has developed ‘’VigiFlow’’, a web-based ICSR [...]  Good pharmacovigilance practices (GVP) are the minimum standard requisite to monitor safety of medicines that is exposed to the public. These are set of procedures that expedite the practice of pharmacovigilance. These practices are used in relation to medicines that are legalized at the national level. These standard procedures ensure authorization holders for an adequate [...]
Good pharmacovigilance practices (GVP) are the minimum standard requisite to monitor safety of medicines that is exposed to the public. These are set of procedures that expedite the practice of pharmacovigilance. These practices are used in relation to medicines that are legalized at the national level. These standard procedures ensure authorization holders for an adequate [...]  A Data Clarification Form (DCF) or Data Query Form (DQF) is a questionnaire specifically used in clinical research. The DCF is the primary data clarification tool from the trial sponsor or Contract Research Organization (CRO) towards the investigator to clarify discrepancies and ask the investigator for clarification. The DCF is part of the data validation process in [...]
A Data Clarification Form (DCF) or Data Query Form (DQF) is a questionnaire specifically used in clinical research. The DCF is the primary data clarification tool from the trial sponsor or Contract Research Organization (CRO) towards the investigator to clarify discrepancies and ask the investigator for clarification. The DCF is part of the data validation process in [...]  Documentation plays a vital role in clinical research. It validates how authentic the research data was collected and verify the result of data. There are many stakeholders in clinical research-participants, sponsor, regulatory authority, ethics committee each playing an integral role for thru drug to come in the market for sale. In clinical research “if is [...]
Documentation plays a vital role in clinical research. It validates how authentic the research data was collected and verify the result of data. There are many stakeholders in clinical research-participants, sponsor, regulatory authority, ethics committee each playing an integral role for thru drug to come in the market for sale. In clinical research “if is [...]  Firstly, we must grasp that the phrase used for clinical trial patient is preferred as participant, they are voluntarily participating in the study and may be healthy or diseased volunteers. By voluntariness it is meant they can withdraw from the study at any given point of time. In clinical trial, the safety of the participant [...]
Firstly, we must grasp that the phrase used for clinical trial patient is preferred as participant, they are voluntarily participating in the study and may be healthy or diseased volunteers. By voluntariness it is meant they can withdraw from the study at any given point of time. In clinical trial, the safety of the participant [...]  Firstly let us understand what is clinical trial Clinical trial in simple terms validates the safety and efficacy of the drug to enter the market for sale.Clinical trial undergoes various phases increasing the number of participant to the next step. Epidemic-The occurrence of more cases of a disease that would be expected in a communt [...]
Firstly let us understand what is clinical trial Clinical trial in simple terms validates the safety and efficacy of the drug to enter the market for sale.Clinical trial undergoes various phases increasing the number of participant to the next step. Epidemic-The occurrence of more cases of a disease that would be expected in a communt [...] 
