Good pharmacovigilance practices (GVP) are the minimum standard requisite to monitor safety of medicines that is exposed to the public. These are set of procedures that expedite the practice of pharmacovigilance.

These practices are used in relation to medicines that are legalized at the national level.
These standard procedures ensure authorization holders for an adequate quality system in monitoring of the licensed medicines; maintain pharmacovigilance data and its documentation and competent staff to carry out these duties.
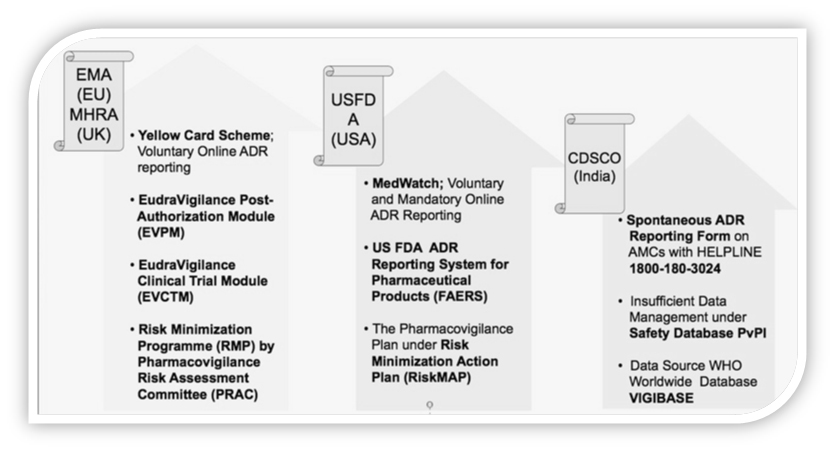
Globally GVP is governed by different organization like MHRA in United Kingdom, EMA in European Union, HPRA in Ireland, SFDA in Saudi Arabia, USFDA in USA and CDSCO in India.
The basic modules of pharmacovigilance for medicines are although the same but there are some variations across the nations in aspect of methods that are used in the monitoring of safety of medicines.
The European Medicines Agency’s Expectations for Good Pharmacovigilance Practice
According to EMA, GVP assists in Monitoring of Compliance and Pharmacovigilance Inspections, Risk Management Plan (RMP) and encompasses the responsibilities of the qualified person responsible for pharmacovigilance (QPPV) and the back-up procedure to apply in their absence, Eudravigilance system and description of electronic reporting.
In 2012, the EMA presented specific sets of principles and processes drawn to pursue further improvements in GVP. As a regulatory authority of the European Union (EU), they are tasked with overseeing post-marketing activities.
Good pharmacovigilancepractices (GVP) are a set of measures drawn up to facilitate the performance of pharmacovigilance in the European Union (EU). GVP apply to marketing-authorisation holders, the European Medicines Agency (EMA) and medicines regulatory authorities in EU Member States. They cover medicines authorised centrally via the Agency as well as medicines authorised at national level.

The Food and Drug Administration’s Expectations for Good Pharmacovigilance Practice
These activities are undertaken with the goal of identifying adverse events and understanding, to the extent possible, their nature, frequency, and potential risk factors.
Although the FDA has expectations in relation to reporting serious adverse drug reactions (ADR), there are not mandatory requirements on the specifics of post-market surveillance.
The main focus of the FDA regarding GVP is guidance on the documentation of safety signals and development of high-quality case reports.
USFDA issued concepts on conducting premarketing risk assessment, developing and implementing risk minimization tools. Premarketing Guidance focuses on risk assessment during late stage clinical development.
The FDA also recommends exploring causal relationships between the use of a drug and the ADR, and using data-mining to identify potential product-event combinations.
In year 2013, India’s contribution to WHO–UMC’s global drug safety database (Vigibase) was 2%. India was 7th in position among top 10 counties contributing to global drug safety database. Among Asian countries, India is the only country having more than 1 lakhs ICSRs in Vigibase.