Softwares like Argus, ArisGlobal, and PvNET are used in Pharmacovigilance. VigiFlow, VigiBase used in post marketing surveillance
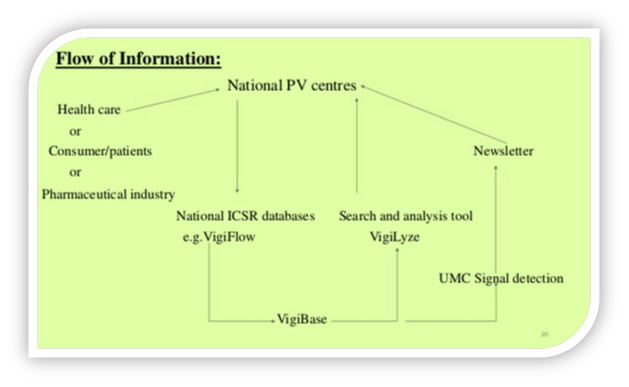
VigiBase is a WHO’s global Individual Case Safety Report (ICSR) database that contains ICSRs submitted by the participating member states enrolled under WHO’s international drug monitoring programme.
Uppsala Monitoring Centre (UMC) in collaboration with ‘’Swissmedic’’ has developed ‘’VigiFlow’’, a web-based ICSR management system. VigiFlow functions as a national ICSR database management system and analysis tool, through which cases are sent to UMC.
Oracle: Argus Safety Database
Oracle Argus is a market leading pharmacovigilance database. The main features of Argus database include global case processing, signal detection, detailed analytics, electronic case intake and electronic expedited reporting in both E2B(R2) and E2B(R3) formats, risk management, periodic reporting and submissions; as well as the capacity to hold large volumes of cases.
Defining features for Oracle Argus safety database include its long history in the pharmaceutical industry, brand awareness and configuration options. It is one of the more complex systems available and therefore we feel better suited to large pharma companies with high volumes as safety data and with the resources to invest in its implementation and maintenance.

Aris Global: ARISg/LifeSphere Safety
ArisGlobal’sLifeSphere Safety (otherwise known popularly as ARISg) is another market-leading pharmacovigilance database with a long and well-established heritage in the industry. It provides comprehensive suite of functionality and tools as well as additional modules and interfaces covering other aspects of pharmacovigilance as well as regulatory, medical affairs and clinical solutions.
Other systems
All safety database systems including those outlined above, PV Works, SafetyBase Interchange, Veeva Safety, etc. offer the same basic functionality and data handling capabilities to support compliance in pharmacovigilance critical activities of ICSR management.
The choice of the database routinely comes down to key stakeholder preference based on prior experience, cost, hosting options and compatible extra features (optional or inclusive as standard).
No one system is superior to all others, all systems seem to have their passionate followers and battle weary sceptics and the success or failure of any system may ultimately come down to the implementation, management, training and oversight of the system rather than which database solution was selected in the first place.

VigiBase is the unique WHO global database of individual case safety reports (ICSRs). It is the largest database of its kind in the world, with over 20 million reports of suspected adverse effects of medicines, submitted, since 1968, by member countries of the WHO Programme for International Drug Monitoring. It is continuously updated with incoming reports. Read more on common concepts and terms here
Alongside its data management and quality assurance tools, the VigiBase system is linked to medical and drug classifications such as WHO-ART, MedDRA, WHO ICD, and WHODrug. These classifications enable structured data entry, retrieval and analysis at different levels of precision and aggregation, which are vital in order to enable effective and accurate analysis.
VigiFlow
VigiFlow is a web-based individual case safety report (ICSR) management system that is available for use by national pharmacovigilancecentres of the WHO Programme for International Drug Monitoring.


VigiFlow supports the collection, processing and sharing of data of ICSRs to facilitate effective data analysis.
VigiFlow is compliant with the international ICH E2B standard and maintained by UMC in Uppsala, Sweden.VigiFlow is available to national pharmacovigilancecentres, but it is not mandatory to use for reporting to VigiBase.
UMC charges a licence fee for VigiFlow, determined by the World Bank Atlas method.