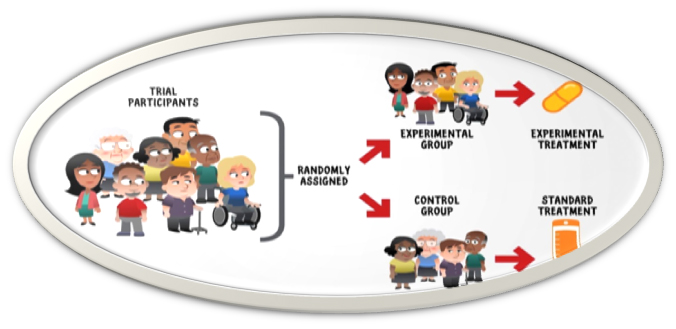
Firstly, we must grasp that the phrase used for clinical trial patient is preferred as participant, they are voluntarily participating in the study and may be healthy or diseased volunteers. By voluntariness it is meant they can withdraw from the study at any given point of time.
In clinical trial, the safety of the participant is paramount over the study protocol, if at any time the data conclude that the harm to the participant is much more that the benefit, the participant can be removed from the study and trial will be prematurely terminated.
Participants in clinical trial have rights and they are protected under law while participating in a clinical trial. All clinical trial are reviewed and approved by regulatory authority and ethics committee.

Regulatory authorities are bodies having the power to regulate. In the ich gcp guideline the expression regulatory authorities includes the authorities that review submitted clinical data and those that conduct inspections. These bodies are sometimes referred to as competent authorities are instituted by the government of the country
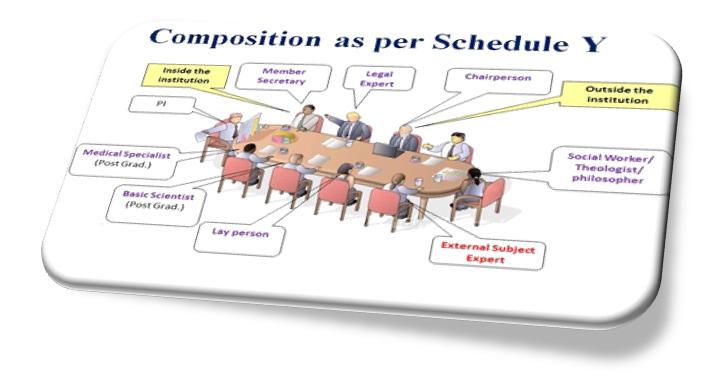
Ethics committee is an independent body (a review board or a committee, institutional, regional, national, or supranational), constituted of medical professionals and non-medical members, whose responsibility it is to ensure the protection of the rights, safety and well-being of human subjects involved in a trial and to provide public assurance of that protection, by, among other things, reviewing and approving/providing favourable opinion on, the trial protocol, the suitability of the investigator(s), facilities, and the methods and material to be used in obtaining and documenting informed consent of the trial subjects.
Informed Consent by Participant
Informed consent is the process of giving clinical trial prticipants all facts about the trial. This happens before they agree to take part and during the course of trial. Informed consent includesdetails about the treatments and tests that participant undergoes and benefits/risks part of the trial.
Before participants decide to enroll in a clinical trial, study team that presents the key facts of the study. If participant agree to part in the trial, will be asked to give consent.

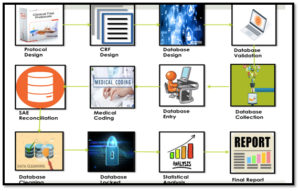
Safety Reporting In Clinical Trial

Clinical trial related injury and serious adverse events are major area of concern.in all such scenario the investigator is responsible for medical care of trial participants and also ethically bound to report events as per country regulatory authority rules and regulations.
The trial sponsor is responsible for ongoing safety evaluation of the investional product, reporting and compensationg the participants in case of any saes.
The ethics committee and regulatory authority of the country are to uphold the principles of beneficience, justice, respect and non-maleficence.